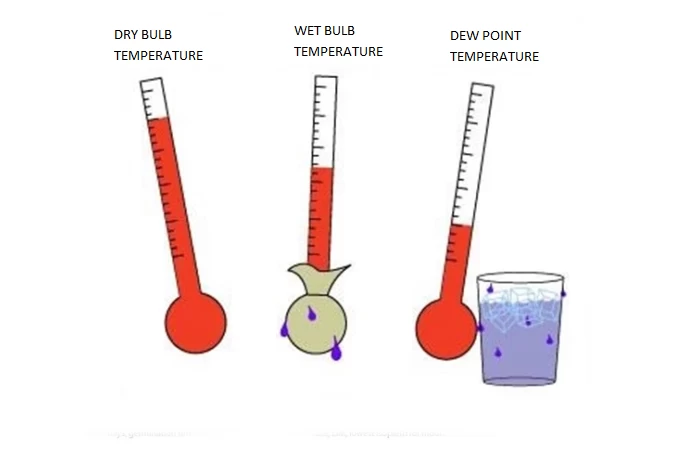
Water in the form of water vapour is an unavoidable component of the air in the environment where we all live and work. At normal humidity levels (35% - 60%) water vapour works on the human body positively, by providing a sense of comfort and euphoria.
Air is able to hold a limited amount of water vapour. The amount of water vapours that atmospheric air can hold in its mass is directly influenced by the ambient temperature.
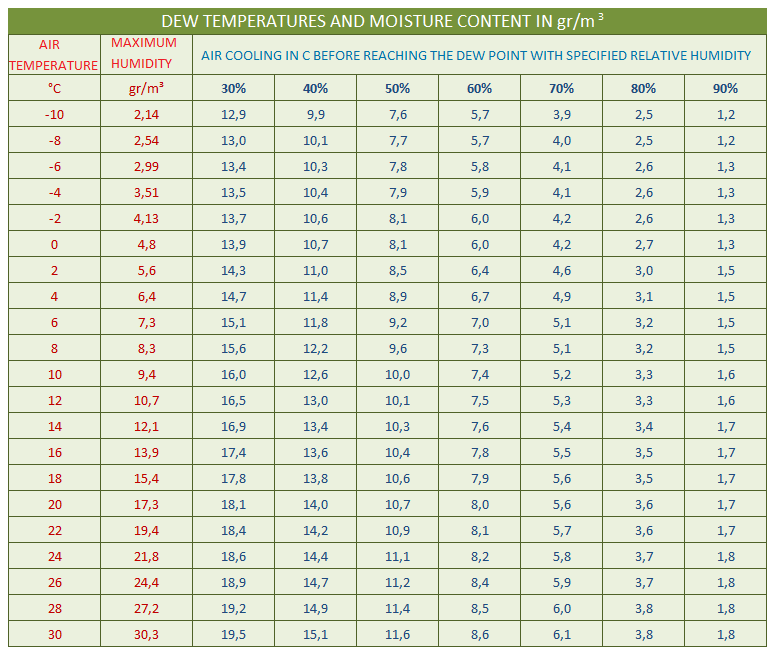
That means, the higher the temperature is, the greater amount of water vapour the air can hold in its mass until it reaches the maximum point of water vapour content, which is characterised as the air's saturation pressure of water vapour. This is easily seen by looking at the first two columns of the table below. It can be seen that for a temperature of 14ºC the air has a maximum potential to hold 12.1 g/m³ of water vapour mass, while for a temperature of 20ºC it has a maximum potential to hold 17.3 g/m³ of water vapour mass.
A key reference is the measurement and visualization of the relative humidity of the room of our interest. Relative humidity is defined as the percentage ratio of the amount of water vapour present in a given volume of atmospheric air to the maximum amount of water vapour that could be present (or that could be retained in its mass) in the corresponding volume of atmospheric air and at a given ambient temperature. Ultimate humidity is the actual amount of water vapour present in the air and is measured in g/m³.
Dew point or dew temperature is the function of the difference between the indoor and outdoor temperature and relative humidity of the room where the condensation of water vapour occurs. This is the liquefaction of the internal water vapour contained in the mass of atmospheric air, which cannot be seen with the naked eye, and corresponds to the temperature at which the saturation pressure of the air in water vapour is observed (see table below).
The higher the relative humidity is, the higher the dew point we see, as a result it gets to the temperature of the room where we observe and the sweating of walls and windows.
This explains why the condensation phenomenon and consequently mould on building elements occurs mainly in kitchens and bathrooms. Same temperature may exist between rooms in a house, but more water vapour (500 to 1 000 g/h from cooking and 1 000 to 3 000 g/h from showering) is produced in the kitchen and bathroom due to specific activities than, for example, we are not doing any other activity than the usual (50g/h water vapour by breathing). Thus, for the same ambient temperature (e.g., 22ºC), the relative humidity in one room may be 80% (e.g., bathroom) and 50% in another (e.g., bedroom).
Studying the table below in relation to the example we see that in one room (bathroom) with a relative humidity of 80% the condensation effect will occur at 18.4ºC (just 3.6 degrees of cooling) while in the other room (bedroom) for the condensation effect to occur the temperature must drop from 22ºC to 11.1ºC (10.9 degrees of cooling). It is concluded that liquefaction and condensation occur on the surfaces of the room which have a temperature lower than the dew point.
As a function of an ambient temperature and a specified percentage of humidity from the table, the dew point temperature can be found based on the cooling of the air indicated in the relative humidity columns.
Example: To determine the dew point, i.e., the beginning of the occurrence of water vapour condensation in a room with a temperature at 20°C and a relative humidity of 70%, we subtract from the temperature the cooling value which is in the table in the 70% column in the 20°C temperature row, i.e. 5.6°C. So, 20 - 5.6 = 14.4°C is the dew point or otherwise the beginning of the condensation effect.
Given an ambient temperature and a specified humidity percentage from the table, the dew point temperature can be found based on the cooling of the air listed in the relative humidity columns.
Example: To determine the dew point, i.e. the beginning of the appearance of water vapor condensation in a room with a temperature of 20°C and a relative humidity of 70%, we subtract from the temperature the cooling value found in the table in the column of 70% on the line of temperature of 20°C, i.e. 5.6°C. So 20 - 5.6 = 14.4°C is the dew point or the beginning of the condensation effect.
A useful tool for measuring the dew point can be found by clicking here
The above phenomenon is observed in the majority of private buildings and should be addressed with a specialized study and implementation proposal. Many people are confused and attribute the causes to moisture coming from the roof when this is not the case. On the contrary, the application of a waterproofing layer can have a negative effect by aggravating the phenomenon.
The phenomenon of condensation of water vapour and the creation of mould in the interior is due to incomplete or no thermal insulation of the roof or wall.

The correct treatment of this phenomenon consists in the installation of thermal insulation on the roof and walls and the interior painting with anti-fog and thermo-reflective paints. Particular attention should be paid to both the quality of the materials and the system to be applied. Prefer certified systems based on ELOT and not patents.
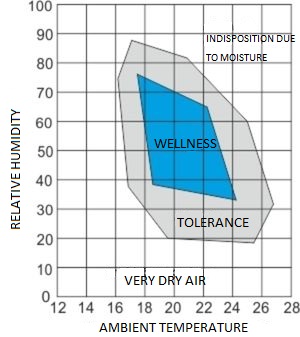
Below is the relevant diagram which shows the temperature and humidity connection for better living conditions.